Audrey Shafer discusses the intellectual and personal inspiration behind the poem "Medicine," featured in Stanford Medicine's year-end video.
Category: Cancer
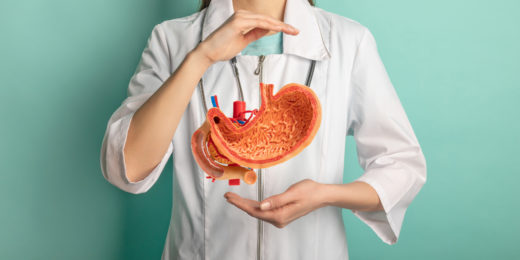
Stomach cancer hits Asian populations harder
Over the last six decades in the U.S., gastric, or stomach, cancer rates have plummeted. But around the world, gastric cancer remains a leading cause …
A 2022 recap: Most read, most viewed and most popular on social media
As the year comes to a close, we're sharing the most-read stories, most-viewed videos and most popular stories on social media of 2022.
Exploring the ordinary and extraordinary in end-of-life care, death
Stanford physician Samuel LeBaron discusses his book, which covers death and how to prepare for and receive end-of-life care.
Stanford Medicine supports Ukrainian visiting scholars
To learn the latest in radiation oncology, Ukrainian scholars visit Stanford Medicine for three months — away from the rubble of the war.
How menthol cigarette ads target Black people, women and teens
As FDA weighs a ban on menthol in cigarettes, study shows how the tobacco industry targeted products to women, teens and Black people.
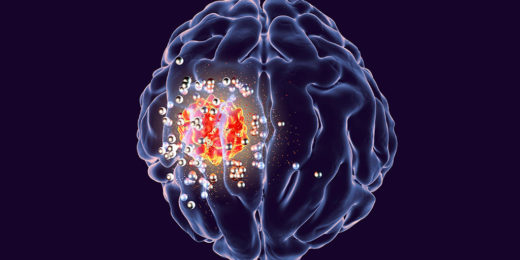
Wireless implant could help remove deadly brain tumors
Brain tumors are among the most deadly and difficult-to-treat cancers. Glioblastoma, a particularly aggressive form, kills more than 10,000 Americans a year and has a …
Cancer-detecting wearable may offer better way to monitor tumors
Researchers from Stanford have developed a wearable sensor to monitor the size of tumors, which could assist new cancer drug evaluations.
Pap smears, be gone? Using menstrual blood to detect HPV
Researchers have created a menstrual pad that can passively help detect HPV, potentially offering a screening method other than pap smears.
Molecules, shmolecules. Why should you care?
We explore the most basic molecular elements of human biology in the lead story for the latests issue of Stanford Medicine magazine.
Coming full circle with extrachromosomal DNA, cancer and Ptolemy
Research into the destructive influence tiny DNA circles have on cancer presents endless ideas for clearly describing groundbreaking science.
Building a cancer community through BLACC
A group of Black women work toward a peer navigation program to help other Black women survive breast cancer.
Stanford Medicine magazine explores the molecules within us
Stanford Medicine magazine explores the molecules behind human biology and how understanding them fuels medical discoveries and innovations.
Data science could help tailor cancer therapy
Researchers are using data science to home in on therapies that will work best for specific patients, advancing precision oncology.
Mobile app helps detect skin cancer in older patients
Scientists used a mobile app to screen elderly patients for potential skin cancer lesions, pointing to the value of digital health tools.
Routing cancer cells to the right path may boost treatment
Researchers at Stanford Medicine discover a certain molecule renders a type of cancer cell more susceptible to treatment.