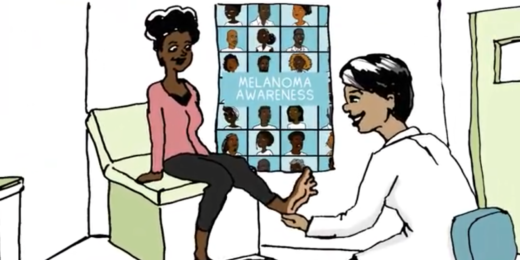
While melanoma rates have leveled off for most of the United States, Black and Latino communities are at a higher risk for the disease.
Category: Cancer
Stanford residents aim to make clinics more sustainable
Stanford Medicine resident and collaborators spearhead an effort to decrease waste from dermatology clinics.
Stanford doctor helps pediatric cancer patients evacuate Ukraine
A Stanford doctor traveled to Poland to help pediatric cancer patients evacuate from Ukraine and receive care.
From loss comes hope: Pediatric brain tumor treatment shows promise
Research from early clinical trials of pediatric glioma patients shows that altered immune cells can fight the deadly brainstem tumor.
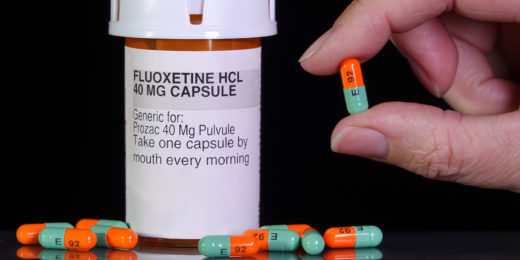
Can Prozac fight brain cancer?
The common antidepressant Prozac melts away glioblastoma tumors in laboratory mice, suggesting possible treatment for the deadly cancer.
Top 5 Scope stories of 2021
From the genetics of COVID-19, to cancer, to tonsils, this story is a wrap up of Scope's most read stories of 2021.
Bill, supported by Stanford doctor, guards against hepatitis
A Stanford Medicine doctor helped write and support legislation to enable free hepatitis B and C screenings for those who request it.
Unleashing the immune system to fight brain cancers
Neurosurgeon Michael Lim studies how to unleash the immune system to attack a type of brain cancer called glioblastoma.
How ovarian cancers evade the immune system
A common ovarian cancer evades detection by convincing nearby immune cells to treat it as a developing fetus.
How to beat cancer? Find the genes that help it hide
Stanford Medicine researchers conducted an experiment to find new genes that, when knocked out, boost immune cells' cancer-killing ability.
Blood test predicts chances of lymphoma relapse after therapy
Stanford Medicine Scientists have devised a blood test to predict some cancer relapses after patients have already been treated.
A better COVID-19 vaccine?
A new way to deliver mRNA as a COVID-19 vaccine may avoid side effects and increase customization to prevent infection.
Keeping treatment-resistant skin cancer cells in check
Anthony Oro is devoted to understanding the origin of basal cell carcinomas. Now he's found how some become resistant to a common treatment.
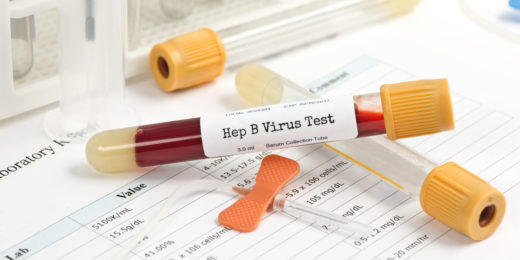
Universal hepatitis B screenings can save lives and cut costs, study says
Stanford researchers find that screening all adults in the United States one time for hepatitis B could save money and lives.
New findings expand hopes for a stem cell cancer ‘vaccine’
Induced pluripotent stem cells share proteins with some cancers. The cells can be used as a vaccine to prevent pancreatic cancers in mice.
Why many stage 3 colorectal cancer patients skip chemo
As risk factors such as no health insurance and low income accumulate, colorectal cancer patients are less likely to finish chemotherapy.