We've all heard the saying that "It takes a village to raise a child." Some scientific problems, like identifying the human skeletal stem cell, require a similar group effort.
Now, a group of interdisciplinary scientists at Stanford, including stem cell researcher and plastic and reconstructive surgeon Michael Longaker, MD, and assistant professor of surgery Charles K.F. Chan, PhD, along with a cadre of others, have identified the cell line responsible for making human bone, cartilage and the spongy bone interior called the stroma.
The discovery is likely to lead to significant advances in our understanding of human skeletal development and our ability to regenerate damaged skeletal tissue.
The researchers reported their findings in Cell. (Medical student Gunsagar Gulati, MD, instructor Rahul Sinha, PhD, and research assistant Justin Vincent Tompkins share first authorship of the study with Chan.)
From our release:
Skeletal regeneration is an important capability for any bony animal evolving in a rough-and-tumble world where only the most fit, or the fastest-healing, are likely to survive very long into adulthood. Some vertebrates, such as newts, are able to regenerate entire limbs if necessary, but the healing ability of other animals, such as mice and humans, is more modest. Although humans can usually heal a bone fracture fairly well, they begin to lose some of that ability with age. And they are completely unable to regenerate the cartilage that wears away with age or repetitive use. Researchers have wondered whether the skeletal stem cell could be used clinically to help replace damaged or missing bone or cartilage, but it’s been very difficult to identify.
Although the mouse skeletal stem cell was identified by Chan and Longaker in 2015, its human counterpart was more wily. Altogether it took the group about ten years to identify the human skeletal stem cell, which is distinct from another category of cells called mesenchymal stem cells that can generate skeletal components as well as fat and muscle. As Chan explained:
Mesenchymal stem cells are loosely characterized and likely to include many populations of cells, each of which may respond differently and unpredictably to differentiation signals. In contrast, the skeletal stem cell we’ve identified possesses all of the hallmark qualities of true, multipotential, self-renewing, tissue-specific stem cells. They are restricted in terms of their fate potential to just skeletal tissues, which is likely to make them much more clinically useful.
Excitingly, human skeletal stem cells can be isolated not just from developing bone and healing fractures, but they can be generated in the laboratory by reprogramming specialized cells in human fat to assume a skeletal fate.
"Every day, children and adults need normal bone, cartilage and stromal tissue. There are 75 million Americans with arthritis, for example," Longaker said. "Imagine if we could turn readily available fat cells from liposuction into stem cells that could be injected into their joints to make new cartilage, or if we could stimulate the formation of new bone to repair fractures in older people."
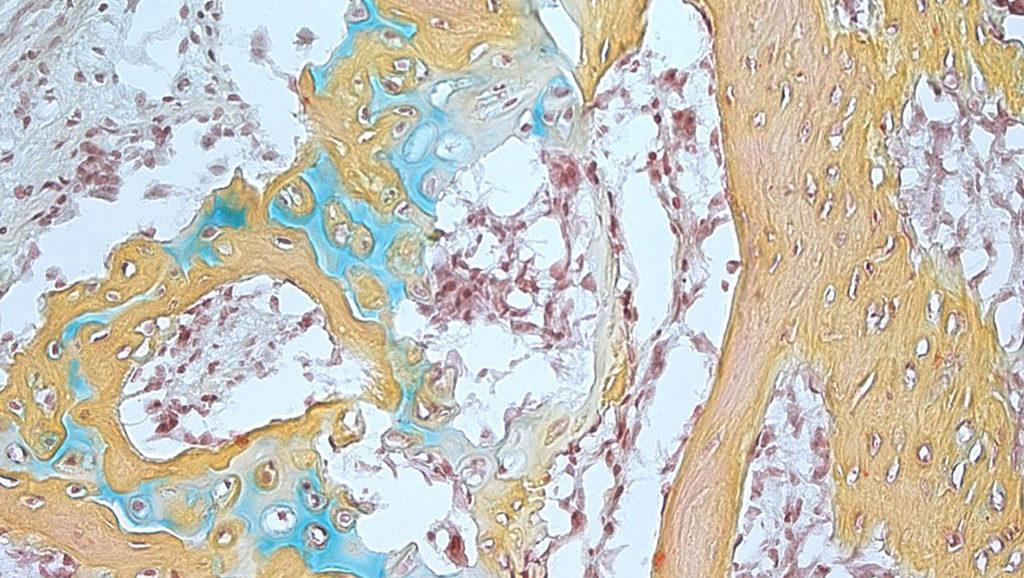
Photo of small bone structure arising from the human skeletal stem cell that contains cartilage (blue), bone marrow (brown) and bone (yellow) courtesy of the Chan and Longaker laboratories