The oxygen we inhale, combined with the food we eat, generates the energy we need to live, think and blog.
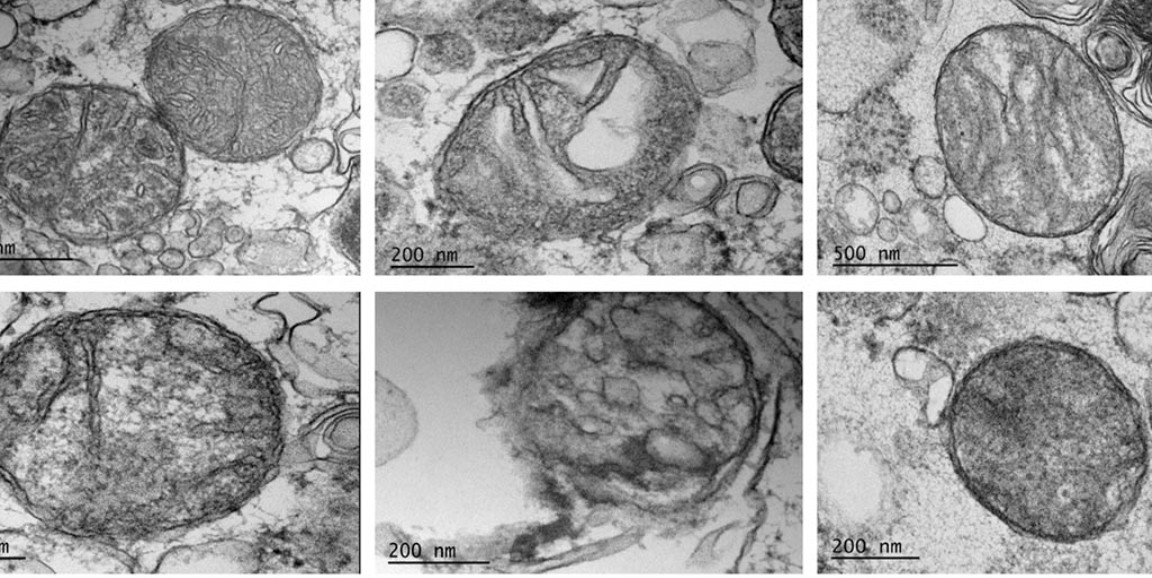
The little machines that transform food and oxygen into a form of energy the body can use are called mitochondria. Let's give a shout-out to the minuscule magicians performing this trick, known as respiration.
They've been hanging out inside the cells of all animals and all plants for a billion years or so -- long enough to have become permanent, mission-critical residents. They can't live without us, and we can't live without them. Our merger is a textbook case of symbiotic teamwork.
But this symbiosis has its downside, as I wrote in my article, "Burnout in Brain City," which appears in the new issue of Stanford Medicine magazine:
The downside of respiration is that it inevitably generates nasty byproducts called free radicals, which not only are harmful to the mitochondria but can also damage cells generally -- not to mention deprive them of the energy they need to function properly. That cell damage, in turn, can set off a cell-suicide program called apoptosis. When things go wrong with mitochondria, they cough out far larger amounts of free radicals, and then things can go very wrong with us, too.
That emphatically goes for our brains, where about one-fifth of all the energy our bodies generate gets consumed. "Burnout in Brain City" describes the findings of Stanford scientists who've implicated mitochondrial malfunction in a broad spectrum of neurodegenerative disorders from Alzheimer's to Parkinson's to Lou Gehrig's disease and more.
Teams led by Stanford neuroscientist Xinnan Wang, MD, PhD, and translational drug explorer Daria Mochly-Rosen, PhD, have not only tracked down some important ways in which faulty mitochondria spur the generation and progression of all these conditions, but identified new kinds of drugs that could lead to their amelioration, cure or even prevention.
Images of healthy and damaged mitochondria by Amit Joshi