Ask any high school biology student to draw a picture of a mammalian cell and they'll likely start with a rough circle (the cell membrane) enclosing the nucleus, mitochondria and other subcellular structures necessary for life.
But I'm betting that most budding illustrators would omit a singular stubby protrusion, called the primary cilia, present on nearly all our cells. Even though the primary cilia was first identified over 400 years ago, we had no idea what it did until relatively recently.
In the past 20 years, researchers have discovered that primary cilia play a critical role in cell signaling and recent findings have shed light on a connection between the once-mysterious cellular appendage and obesity.
In 2019, molecular biologist Peter Jackson, PhD, showed that a molecule called FFAR4, which is found on the primary cilia of cells called preadipocytes stimulates the formation of fat cells that squirrel away saturated fats from the blood. FFAR4 is the receptor for omega-3 fatty acids, commonly found in fish oils, and findings from the research linked omega-3 fatty acids to the creation of new, healthy fat cells.
Now Jackson and developmental biologist Seung Kim, MD, PhD, have learned that signaling through FFAR4 also enhances the ability of beta cells in the pancreas to secrete insulin in response to a sugary snack. It appears to function similarly to the mechanism exploited by current diabetes drugs.
The discovery, which was published recently in Genes and Development, reveals the molecular details of the relationship between diabetes and obesity, and could lead to the development of new diabetes drugs that can amplify the action of existing medications.
"This is a seminal finding that has implications for all types of diabetes," said Kim, who directs the Stanford Diabetes Research Center. "To find that FFAR4 augments the increase in insulin secretion that occurs in response to diabetes drugs is very exciting."
Regulating blood sugar levels
In healthy people, insulin (which is made by beta cells in the pancreas) stimulates the uptake of glucose from the blood after a meal. Another hormone, glucagon (which is made by alpha cells in the pancreas), mediates insulin release when blood sugar levels start to fall. Together the two hormones ensure a relatively constant blood sugar level as healthy people fast and gorge throughout the day. But people with Type 2 diabetes struggle to make enough insulin to adequately control the glucose levels in their blood.
Jackson, Kim, and postdoctoral scholar Chien-Ting Wu, MD, PhD, knew that a family of signaling receptors called G-protein coupled receptors, or GPCRs, are important for beta cell function, and therefore insulin secretion. One GPCR in particular, the receptor for a molecule called GLP-1 that is produced in the intestines after a meal and stimulates insulin output, is the target of many existing diabetes drugs.
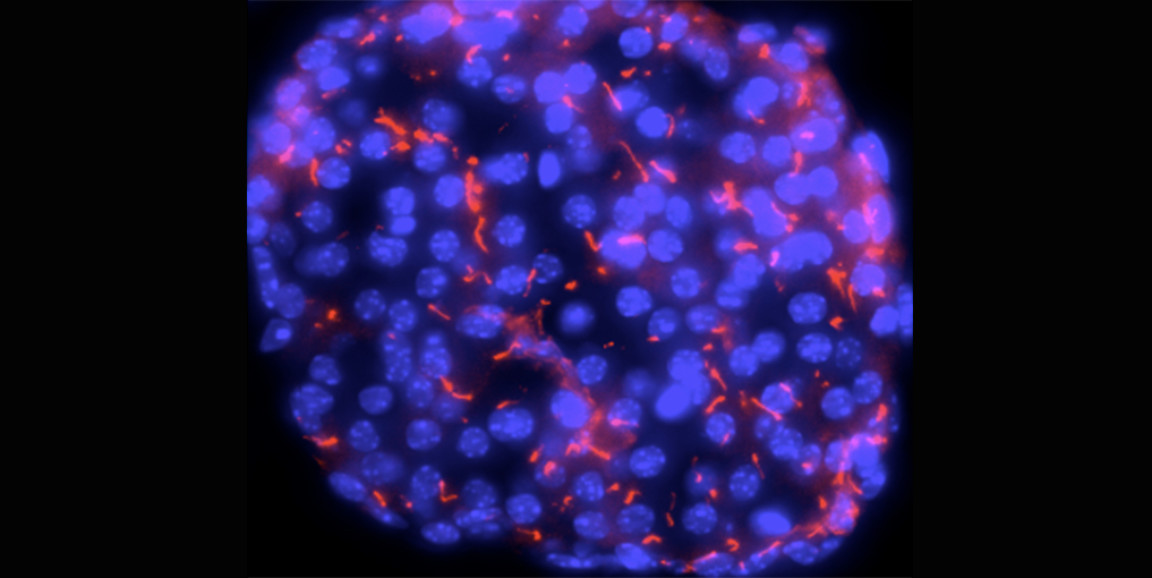
So the researchers posed two new questions: Which, if any, GPCRs are found in the primary cilia of beta and alpha cells in the pancreas? And, if present, do they play a role in insulin and glucagon secretion?
By analyzing mouse beta and alpha cells, they identified several GPCRs on the primary cilia, one of which was Jackson's old friend, FFAR4. The team showed that when FFAR4 is stimulated by a mimicking omega-3 fatty acids in the cells, the cells secrete insulin and glucagon in response to glucose. The effect was as strong as that seen with current diabetes drugs.
"It turns out that stimulating FFAR4 works about as well as stimulating the GLP-1 receptor," said Jackson. "And we can combine them to see an even greater effect on insulin secretion."
Blocking the transport of FFAR4 from the cytoplasm into the primary cilia reversed this effect -- confirming the role of the primary cilia in insulin secretion.
"Finding that signaling through FFAR4 augments the signal from GLP-1, and does so through a critical cellular structure, is a really exciting finding that has important biological implications," said Kim.
More to come for primary cilia
Jackson and Kim plan to continue their investigation into the mechanisms downstream of FFAR4 signaling that trigger insulin release. Kim will also explore whether signaling through the primary cilia may play a role in the development of pancreatitis or pancreatic cancer through regulation of cell growth and stability. Both scientists hope their current findings eventually lead to improved diabetes treatments.
"Now we can start to organize our thinking around diabetes and obesity, and how we can best help patients," said Jackson. "If we can learn how to boost the activity levels of current diabetes drugs, or how to overcome the acquired or intrinsic resistance that makes them less active in some patients, we could enhance treatment options."
Photo by Chien-Ting Wu