People with diabetes must plan each meal, snack and insulin dose, a major hassle that may one day be eliminated thanks to discoveries from venom of an unexpected undersea critter: the cone snail.
Diabetics don't make enough insulin to move sugar from their blood into cells around the body; thus, they need to inject the hormone or wear an insulin pump. Insulin delivered under the skin acts quite slowly, meaning patients must calculate the carbohydrates in every snack or meal and give themselves insulin 20-30 minutes in advance. But insulin-like molecules from cone snail venom are inspiring bioengineers to design faster-acting alternatives, with the potential to free patients from the need for close tracking of everything they eat.
Cone snails live in ocean shallows, where they hunt tiny sea creatures, such as worms, other snails, or small fish, using paralyzing venom. The venom, made mostly of neurotoxins, also includes some insulin-like molecules -- the only known example in nature of an animal weaponizing this hormone. Giving a fish a big dose of insulin causes its blood sugar to plummet, making it harder for the fish to escape the hungry snail.
Chemical biologist Danny Chou, PhD, was working at the University of Utah in 2014 when marine biologists there discovered the "venom insulins." He wondered if the animal's odd feature might help solve insulin-dosing challenges in people. So he and his colleagues in Utah investigated further, designing new venom-human hybrid insulins, and have been studying how they work. Chou brought the research with him when he joined Stanford's faculty in 2020.
A paper published March 14 in Nature Chemical Biology shows that the bioengineered insulin molecules have several promising features, including lack of stickiness and "just right" binding with their target receptors. The bioengineered molecules don't stick to each other, which will likely make them much faster-acting when injected, but do interact with the human insulin receptor to activate sugar transport.
"When we started, this was the perfect scenario I wanted to reach," said Chou, senior author of the paper. At first, he thought this outcome was a long shot, figuring there was only about a 5% chance the venom insulins would solve two longstanding problems -- of self-stickiness and receptor specificity -- in insulin engineering. "We know quite a bit about insulin and how it works -- it's been nearly 100 years since it was discovered -- but there are still discoveries like this that you can't predict," he said.
Bioengineering hurdles
Human insulin is a small but mighty hormone, responsible for transporting sugar from the blood into cells and for helping to stimulate cell growth. It is a protein made of two chains of amino acids, the 21-amino acid A chain and the 30-amino acid B chain.
In the pancreas, where insulin is made, the hormone clumps up for storage -- specifically, the B-chains help the insulin molecules to stick together in groups. When a healthy person eats, their blood sugar rises and the pancreas secretes insulin clumps into the bloodstream. Each clump rapidly dissolves into individual insulin molecules that move through the blood to usher sugar into cells.
But when people with diabetes inject insulin under their skin, the clumps stay glommed together for a while, slowing the hormone's actions. (It's like the difference between sprinkling salt in a pot of soup, where it dissolves instantly, versus onto a plate of scrambled eggs, where the crystals glisten back at you.)
In the past, bioengineers tried getting rid of the sticky part of the molecule, shortening human insulin's B-chain by a tiny bit, which eliminated clumping. But the resulting molecule didn't bind to insulin receptors at all.
"It lost all its potency," Chou said. That's why he was intrigued by cone snail venom. Venom insulins lack the sticky region of the B-chain and never clump up. Yet they still powerfully lower blood sugar in fish.
Inspiration from cone snail venom
Chou's team created six kinds of hybrid molecules, scaffolding the likely key features of venom insulins onto human insulin -- only these lab-made molecules lacked the sticky part of the B-chain. The hybrid insulins had small molecular additions to their A-chains, which were cribbed from cone snail venom.
The hybrid insulins didn't stick to each other, the researchers found. And their latest work -- which employs cryo-electron microscopy, a type of structural imaging, to capture images of the hybrid insulins binding with human insulin receptors in high resolution -- shows that four of these hybrids bind quite well to human insulin receptors. The images captured were at such a high magnification that the scientists could see small shifts in individual molecules.
When the hybrid insulins bind to the receptors, they cause the receptor to change shape, triggering other cell signals. The shape changes induced by the hybrid insulins suggest that it may be possible to fine-tune them toward activating just one of the hormone's two main functions: moving sugar out of the blood stream. Even more interesting -- the insulin receptors take on different shapes when bound to hybrid insulins versus human insulin, which gives clues about how the receptor activates different cell-signaling pathways, Chou said.
"Normally, human insulin can make cells bigger and make them divide faster. In the long run, this can raise potential concerns about cancer for people with diabetes," Chou said. Tinkering with the insulin given to people with diabetes could theoretically raise their cancer risk, so it would be a big bonus if the molecular changes in venom-human hybrid insulins didn't trigger excess cell division. "Whenever someone comes out with a new insulin, we want to make sure it doesn't give more growth-promoting signal." The team is continuing to investigate this possibility.
Onwards to the artificial pancreas
The hybrid insulins now need to be tested in animal models of diabetes and in clinical trials in people. If these tests work well, Chou has high hopes for how the fast-acting hybrids could ease the lives of people with diabetes.
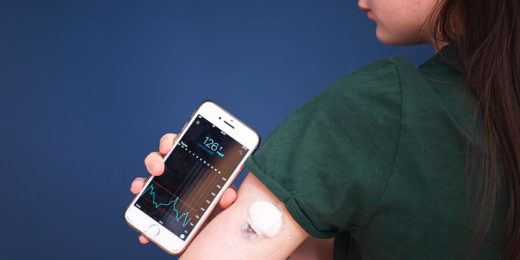
It's already possible for patients to wear a small under-the-skin glucose sensor connected digitally to an insulin pump. These "closed-loop" treatment systems automate many aspects of diabetes care, but are hampered by the slow action of insulin injected under the skin, and they still require patients to count carbohydrates and plan insulin doses in advance of meals.
Chou hopes for a day when patients could instead eat what they want, when they're hungry, and trust their closed-loop diabetes technology to take care of the rest, sensing the rise in blood sugar, then quickly releasing tailored amounts of a fast-acting venom-human hybrid insulin that would soon return the person's blood sugar levels to a healthy range.
Meanwhile, cone snails around the world will still be burrowing in the sand, waiting to attack unsuspecting fish with their powerful venom, never knowing their toxic weaponry has been reengineered to help humans.
Photo by Pet