When Gang Pan first came to Stanford Medicine, he could no longer work or drive and was uncomfortable even venturing out in public because an autoimmune condition had severely restricted his vision and the movement of his eyes.
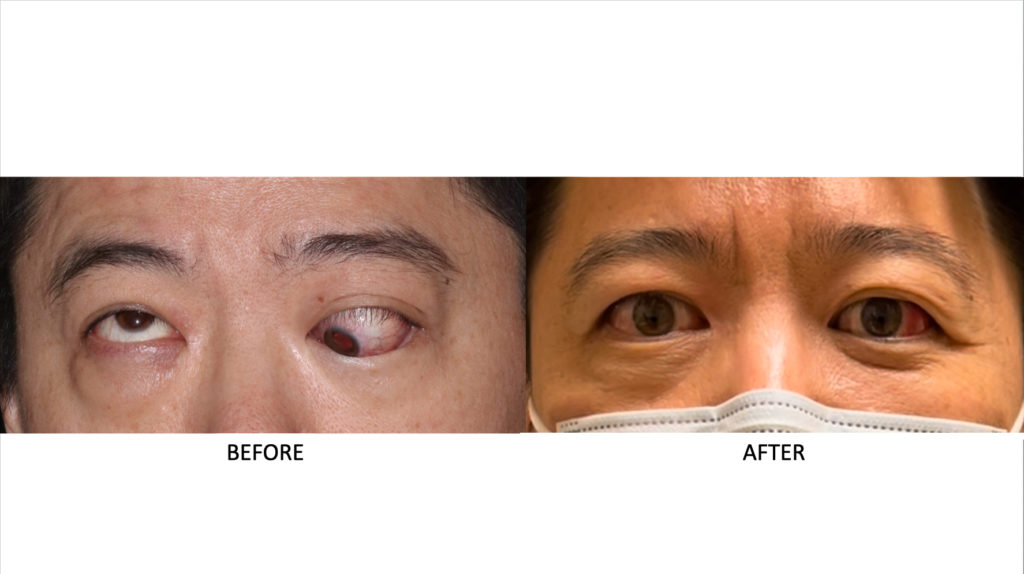
A condition called thyroid eye disease had caused the soft tissue behind his eyes to swell and push one of his eyeballs to point up and the other to point down, rendering him functionally blind.
"I had to cover my eyes to go outside because they were really scary even just to look at," said Pan, who was seen at the multidisciplinary Thyroid Eye Clinic. "As the swelling got worse, it got to the point where I didn't want to go out at all."
In addition to restricting his eyeballs' movement and creating severe double vision, the inflammation also compressed Pan's optic nerves -- which carry images from the eye back to the brain -- affecting his ability to see and discern colors, and threatening permanent blindness.
But in January 2020, Pan became the first patient with sight-threatening thyroid eye disease to be treated with a newly FDA-approved drug called teprotumumab, or Tepezza. After two IV infusions of the drug, Pan regained the ability to move his eyes and see colors and details of objects from afar.
"We were shocked and so excited," said Stanford Medicine assistant professor of ophthalmology Andrea Kossler, MD, who co-led his treatment team. "We did not expect such a dramatic improvement, especially after just two infusions."
Thyroid eye disease: An unpredictable autoimmune disease
Symptoms of thyroid eye disease range from mild complaints like dry eyes or redness to moderate symptoms like bulging eyes and double vision, all the way to the severe symptoms Pan experienced, which can include blindness.
Studies suggest that the disease affects approximately 16 in 100,000 women and 3 in 100,000 men in the United States.
"Thyroid eye disease is a heterogeneous and unpredictable autoimmune condition," said Kossler.
Prior to 2020, there were no targeted or FDA-approved treatments for thyroid eye disease. Patients with advanced forms of the disease received high doses of steroids or radiation to halt inflammation, but these treatments did not reverse the disfiguring or disabling symptoms already caused. Other patients might have to wait months before becoming eligible for surgery to fix bulging eyeballs, double vision or eyelid changes.
New understanding offers hope for treatment
About 10 years ago, researchers uncovered that the insulin-like growth factor-1 receptor, or IGF-1R, played a role in thyroid eye disease. The receptor gloms onto another receptor, called the thyroid stimulating hormone receptor, and forms a complex. Self-attacking antibodies created by the immune system target the receptors and activate that complex.
When this complex is activated, the cells in the eye can turn into fatty tissue and produce chemical messengers that lead to soft tissue expansion and inflammation. The newfound understanding of the importance of the IGF-1R in thyroid eye disease pathogenesis led to the use of teprotumumab, said Kossler. "The recent understanding of the pathophysiology of disease has led to a paradigm shift in the way we treat this condition."
Teprotumumab is a monoclonal antibody -- a protein derived from a single immune cell and copied many times -- that binds to and blocks this IGF-1 receptor. Evidence from two clinical trials showed that 83% of patients with active moderate-to-severe thyroid eye disease experienced a reduction in eye bulging after eight infusions. These clinical trials excluded patients with sight-threatening thyroid eye disease. Therefore, even after teprotumumab gained FDA approval in January 2020, no one knew if it would work for severe, sight-threatening symptoms like those Pan was experiencing.
By then, Pan had been having symptoms for more than 18 months and had tried a variety of unsuccessful treatments, including intravenous steroids and orbital radiation.
"At that point I'd pretty much lost my vision," Pan said. "My right eye could still see, but it didn't operate properly unless I physically moved it with my hand."
Pan's appearance had changed so dramatically that the facial recognition software on his iPhone could no longer identify him.
"This disease feels like you are walking into darkness," Pan said, "deeper and deeper. And when you're in that darkness, you really want to grab anything that can give you hope. Tepezza was like a light showing up -- finally, a hope."
After infusions, relief 'was magical'
Pan said the IV infusions weren't painful and took about an hour. Within 48 hours, his symptoms were already improving. "I had the infusion in the afternoon, and by that second night after, my eyeball movement already felt less restrictive. It was magical."
The improvements were even more striking after a second infusion. "Not only was his eye movement significantly better," Kossler said, "but his visual field tests, his color vision and his visual acuity had all improved."
A repeat MRI showed that the muscles around his eyes had decreased in size considerably, reducing the volume of soft tissue within his orbits and relieving the pressure on his optic nerve, Kossler said.
During the full course of eight infusions, Pan continued to improve, with only minimal side effects. Because IGF-1 receptors are found throughout the body, including in the ear and gut, some patients can experience adverse effects from teprotumumab, including diarrhea, hyperglycemia, muscle problems, hair and skin changes, and hearing loss.
After the treatment, Pan's disease had stabilized enough for him to undergo surgery to correct the residual eye bulging and restricted muscle movements. Pan now has 20/20 vision and can move his eyes freely.
"It really changed my life, to be honest," Pan told Kossler. "The first few days after getting my vision back, it was like the feeling of first learning how to ride a bike and wanting to ride it every day. You just want to go out and see the world because now you don't even have to cover your eyes. You feel normal again and you can see the world and all its beautiful things."
Ongoing clinical trials test multiple treatment options
Kossler said that early diagnosis and treatment of thyroid eye disease are critical because it's much easier to stop disease progression than to reverse its effects for patients like Pan.
"For years, these patients have been told there's nothing that can be done for them, and that's just not true anymore," Kossler said. "It's important to educate our community and our providers that at Stanford, we have a lot of different options."
Because the condition is an eye disease and a hormone disorder, multiple specialists collaborate to address these patients' needs. Kossler co-directs the Multidisciplinary Stanford Thyroid Eye Disease Clinic with endocrinologist Chrysoula Dosiou, MD, a clinical professor of medicine in endocrinology, gerontology and metabolism.
While ophthalmologists treat the localized eye symptoms, endocrinologists can address the underlying imbalance in thyroid hormone levels. "Prompt correction of thyroid dysfunction is integral to the success of treatment," Dosiou said, "as both hyperthyroidism and hypothyroidism are risk factors for worsening eye disease. Additionally, many therapies for thyroid eye disease increase the risk for endocrine-related side effects."
At the Stanford clinic, Dosiou and Kossler often see patients together, as they did in Pan's case. "It was a very exciting time," Dosiou said, "to see the dramatic changes in his eyes and quality of life. And it is extremely rewarding to see how well he is doing now, after he has completed multiple other treatment steps in his thyroid eye disease journey."
To provide optimal care for these patients, Dosiou and Kossler also work closely with colleagues in radiation oncology, neuro-ophthalmology, thyroid surgery, and rheumatology.
The team is recruiting patients for several clinical trials to continue evaluating teprotumumab and to study other IGF-1 receptor inhibitors that patients might tolerate better or find more convenient. One formulation, for example, may be injected under the skin, while another can be taken orally.
"When you have this disease, you feel almost hopeless, like there's no chance this can be cured," Pan said. "But just walking into the eye clinic, you get this feeling like they really care. They have a sign that says, 'This is a place for healing.' Every time I saw that sign, it moved me and gave me hope."
Photo by Romaset