Unconventional Paths: Stories of Stanford Medicine faculty, researchers and physicians whose journeys into medicine followed nontraditional routes
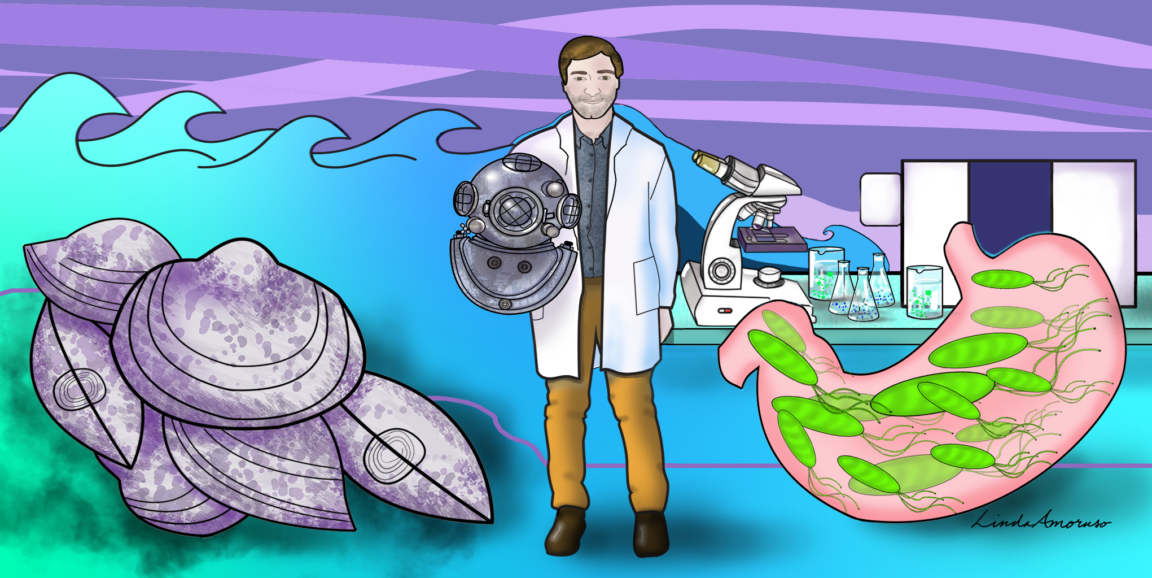
Gurgling. Harsh. Inhospitable. Those three words describe the deepest pits of the oceans -- and the human stomach. Not much, save for some types of bacteria, can survive in either place, and even they need to be highly specialized.
Benedikt Geier, PhD, a postdoctoral scholar at the Stanford School of Medicine, has traversed the two bacterial realms, harnessing a chemical analysis technique that captures the close interactions between bacteria and the worlds they inhabit.
Geier has always been fascinated by microscopic organisms and how they survive in environments where others would surely perish. From inspecting bacteria that dwell in deep-sea mussels to those that live in the stomach and cause ulcers, Geier is peeking into another world, rife with rarely seen interactions.
"The host-microbe relationship provides a window into one of life's fundamental connections -- connections that can be found nearly anywhere, including inside each one of us," Geier said.
At first glance, the deep sea and the stomach couldn't seem more different, but the insights Geier is after start with the same question: How do bacteria colonize, infect and persist in their host?
"It's all governed by a chemical language that we normally cannot see," Geier said. But through specialized tools, Geier is unraveling those questions and deciphering the intimate inner workings of bacteria, and how they can help -- or hurt -- their host.
Bacterial superpowers
Geier pursued his undergraduate degree at Ludwig Maximilian University in Munich, Germany. He earned his master's at the same university, where he studied eye formation in tiny chameleons native to Madagascar. Then Geier pursued a doctorate at the Max Planck Institute for Marine Microbiology, studying deep-sea vents and symbiotic bacteria in a type of deep-sea mussel.
Geier didn't expect to transition from his career as a deep-sea microbiologist to a biomedical researcher. But after years of studying bacteria in deep sea volcanoes, he began to see common threads: namely, the unique survival strategies that underpin the synergistic give-and-take of a microbe and its host.
Organisms everywhere depend on bacterial partners to accomplish all sorts of weird and wild feats: Bacteria help deep-sea mussels use hydrogen as energy, the mouths of blue-ringed octopi are thought to produce paralyzing neurotoxins that they wield against enemies and prey, and bacteria help the bobtail squid glow. None of these would be possible without the quirks of microscopic bacteria that work to their partner's advantage. We humans benefit from that partnership, too. Bacteria in the large intestines aid digestion and regulate metabolism, and bacteria in mucus and on the skin help protect against pathogens.

(Fun fact: Our own cells are outnumbered by our microbial counterparts 1:10.)
But not all relationships are supportive: Bacteria can be antagonistic, too. It's the switch between these two roles that captured Geier's imagination with H. pylori, which is not always a "bad" bacterium.
To investigate, Geier harnesses something called mass spectrometry imaging, which distinguishes the chemical composition of a sample based on its mass and location in the host. That might not seem like more to go on, but it helps shed light on where the bacteria thrive and the molecules they produce, eat and coax out of the host.
In combination with mass spectrometry imaging, Geier uses another microscopy technique that helps draw associations between the bacteria and the chemicals seen with the mass spectrometer.
A toxic mystery
In January, Geier joined the lab of Manuel Amieva, MD, professor of pediatrics and of microbiology and immunology, swapping mussels for the sloshy seas of the stomach, investigating the microbial underpinnings of H. pylori as it teeter-totters from good to bad.
Infection with H. pylori has long been one of the most widespread chronic infections in the world. But with the advent of antibiotics and acid secretion inhibitors, those affected have a cure.
Still, a big question remains.
H. pylori is typically pigeon-holed as a bad bacterium, but that isn't always the case. "H. pylori infects half of the world's human population, yet only about 1% of people develop stomach diseases," Geier said. So, what's the trigger that turns H. pylori bad? "Chemical analysis can help us solve that question."
With mass spectrometry imaging, scientists like Geier can tease out bacterial locations to understand what they do in their natural environment and the communication networks tracked through various chemicals. The hope is, this data can help scientists control the chemicals produced by bacteria, ultimately allowing them to suppress interactions that lead to infection.
"H. pylori colonizes the pits of the stomach glands," Geier said. "But what makes this environment so attractive for the bacterium and what the chemical conversation is between the bacteria and the stomach causing it to become toxic is a mystery."
Scientists don't yet know what happens on a chemical level within the first few minutes or hours after H. pylori meets the stomach glands it infects. Geier is planning to change that.
"Unlocking this black box will help us understand what gives this bacterium an advantage in the harsh environment of the stomach -- a world that not many bacteria can inhabit, much less successfully thrive in," Geier said. "We will begin to understand H. pylori-induced diseases only once we start to ask what's in it for the bacterium."
Read more Unconventional Paths stories here.
Illustration by Linda Amoruso