Standard diagnosis of sepsis relies on a blood test that typically takes days. A Stanford physician is working on an innovation that could change this.
Category: Bioengineering
How thoughts could one day control electronic prostheses, wirelessly
Stanford researchers have shown how to create wireless brain-computer interfaces that could enable amputees to operate thought-controlled prostheses.
Device could help patients test blood ammonia levels at home
After treating a patient with an unusual ammonia metabolism problem, a Stanford researcher assembled a team to reimagine ammonia blood testing.
Addressing the gender gap in health tech
Through a survey, an initiative and a speed-mentoring event, the Stanford Byers Center for Biodesign is taking on gender inequalities in health tech.
‘Instagram-like filter’ labels molecular details in tumor images
Scientists created an algorithm that analyzes a cancer biopsy and pairs spatial information with gene expression to better understand the disease.
Researchers formulate new ultrafast insulin
Stanford University bioengineers are developing a faster-acting formulation of insulin that can help diabetes patients better regulate their blood sugar levels.
COVID-19 adds urgency to synthetic film that aids breathing
The COVID-19 pandemic gives new relevance to a synthetic substance developed by Stanford researchers that could help respiratory patients breathe easier.
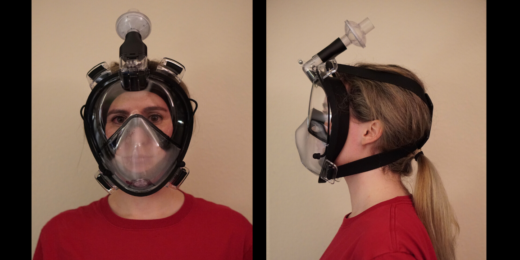
Scientists redesign full-face snorkel mask to combat PPE shortage
Stanford bioengineer Manu Prakash and his team have transformed full-face snorkel masks into reusable personal protective equipment for health care workers.
Motor-powered ankle exoskeleton could make running easier, faster
Stanford bioengineering researchers find that a motorized device that attaches around the ankle and foot can drastically reduce the energy cost of running.
New brain implant device could record activity in thousands of neurons
Stanford University researchers created a device that, if implanted in a brain, could help record the activity of thousands of neurons.
Macular degeneration steals sight. A chip implant may get it back.
In a clinical trial, a tiny prosthetic retinal device invented by a Stanford researcher has proved its potential ability to restore eyesight to the blind.
In the Spotlight: Using engineering to improve patients’ lives
This "In the Spotlight" features Ross Venook, a bioengineer who discusses his career path and his life as a busy father and husband outside of work.
It’s go-time: a doctor and student engineers work to make catheterization easier
A Stanford team has developed a guiding device to help woman self-catheterize, with the goal of improving patient comfort and preventing infections
Under pressure: New technique helps ID bacteria
A stress test helps researchers distinguish between different kinds of bacteria by testing their cell wall strength under pressure.
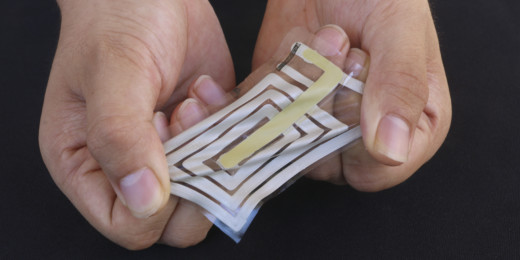
Sticky sensors developed to detect skin’s signals
A new wireless system developed by Stanford engineers detects health indicators like pulse and respiration from the skin via wearable stickers.
Do probiotics live up to the hype? Part II
The conclusion of this series examines the benefits, and drawbacks, of probiotics. Stanford researchers clarify whether probiotics really improve health.