I've written here before about my fascination with the 1966 film Fantastic Voyage and its corresponding novel by Isaac Asimov. The film and book describe the adventures of the crew of a miniaturized submarine called Proteus that was injected into the body of a Very Important Person to hunt down and destroy a potentially fatal blood clot.
The idea of being able to watch a single blood or immune cell going about its day-to-day business inside the body still fills me with wonder.
Now, Stanford radiation oncologist Guillem Pratx, PhD, and postdoctoral scholar Kyungoh Jung, PhD, have come a step closer to making my childhood daydreams a reality. They've devised a way to use a common imaging technology called positron emission tomography, or PET, to watch the movement of a single cell injected into a laboratory mouse in real time. The researchers recently published their results in Nature Biomedical Engineering.
As Pratx explained:
PET scans are one of the most sensitive ways of detecting molecular processes in patients. More than two million PET scans are conducted every year in the United States -- but until now, no one had tried to track the movement of an individual cell using PET.
Labeling a single cell with radioactivity
PET scans rely on the use of radioactive drugs called tracers. These tracers are injected into the body; and they accumulate in areas of high metabolic or chemical activity, which often signal the presence of diseases like cancer or heart disease. Large, whole-body scanners are then used to identify the location of the radioactive signal within the patient.
Until now, however, it's been impossible to label a single cell with enough radioactivity to be detectable within a living animal. That is, until Pratx and Jung hit on the idea of loading a radioisotope onto what are called mesoporous silica nanoparticles.
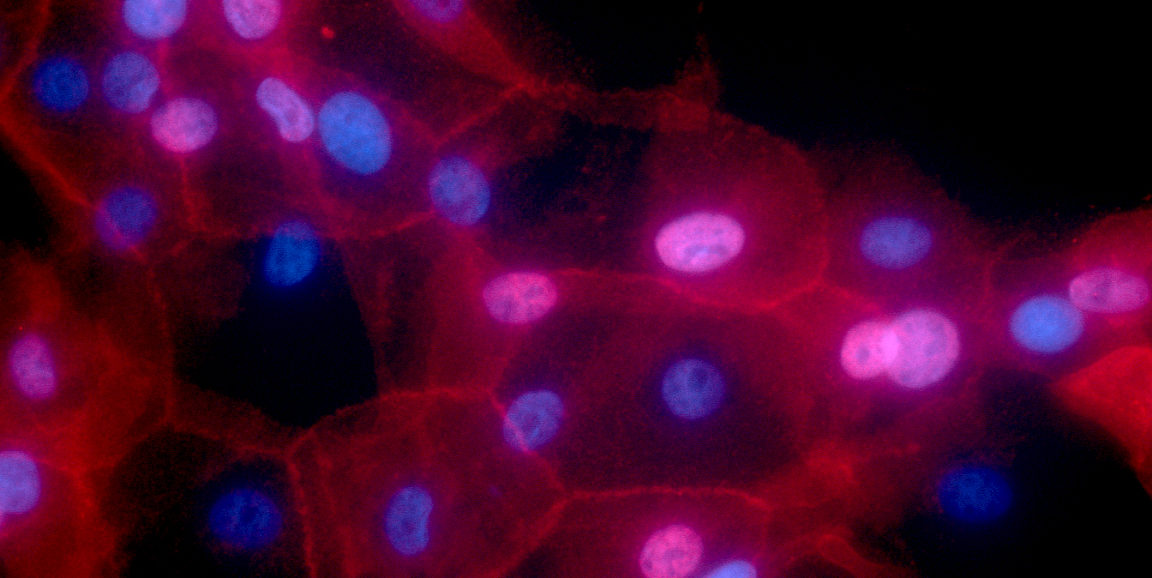
"These particles are like little sponges -- they are very porous," Pratx said. "So we loaded the radioisotope onto the nanoparticles, and then attached those to human breast cancer cells grown in the laboratory."
Tracing a single cell in a mouse
The technique worked, and the researchers were able to trace the movement of a single labeled cell from its injection into the tail vein of a laboratory mouse to its final resting place in the animal's lung. They had to be quick, though. Much like Proteus, the labeled cell zipped along in the blood flow at a clip of five centimeters per second.
Although the technique, which the researchers call cellGPS, still needs to be optimized before it can be used in humans, it's exciting to imagine the future possibilities.
As Pratx explained:
Now we have sensitivity to look at the location and movement history of individual cells, rather than just seeing them at a single point in time. This may be particularly useful for tracking the effectiveness or outcome of cellular therapies, such as cancer-fighting CAR-T cells, particularly in solid tumors. Are they getting to the target tissue? How long does it take? How does the tumor respond? Eventually we hope to have the ability to track multiple individual cells simultaneously.
Isaac Asimov would be astounded. Or maybe he expected it all along.
Image by Ewa Krawczyk, National Cancer Institute \ Georgetown Lombardi Comprehensive Cancer Center, National Institutes of Health