On a recent Saturday morning, my coffee grinder came apart in my hands. Normally, it's a quick beans-lid-grind process, in which I wait until the whizzy, gritty sound reaches the right timbre. This time, as I removed the lid, the machine's plastic housing broke off.
I could see from the grinder's insides how it works. A knob on the lid pokes a (normally hidden) plastic pin, which prods the motor-activating switch, which makes the blades spin. To get it working again, I had to put everything together exactly as before. I fiddled with pin alignment, then gingerly put the housing into place. Gadget restored!
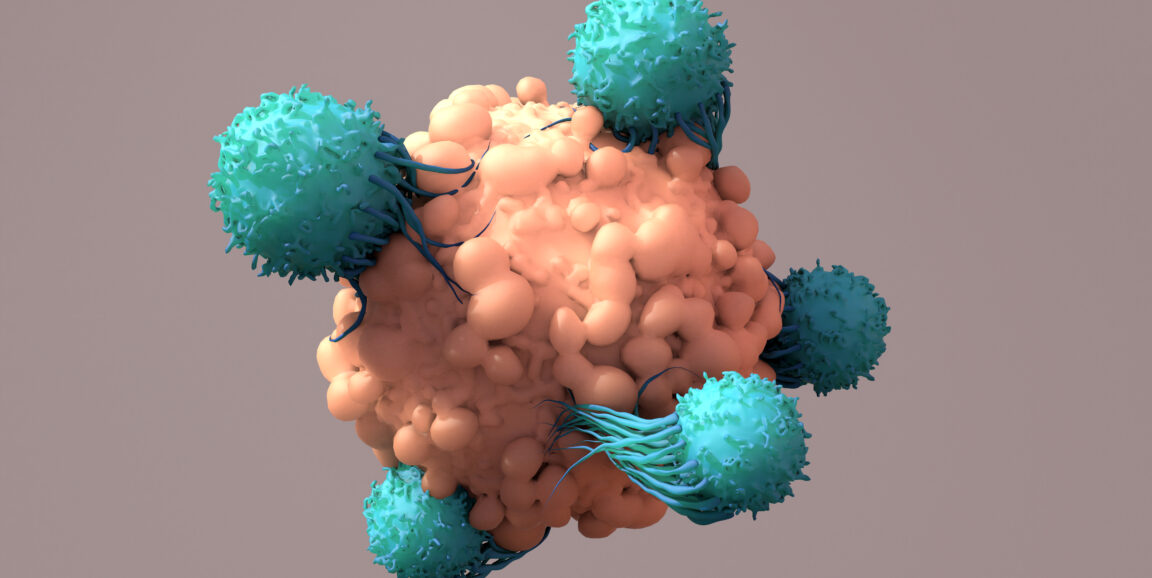
I relay all this to explain why I was gobsmacked by a scientific paper that crossed my desk recently. Stanford Medicine cancer researchers Robbie Majzner, MD, Aidan Tousley and their colleagues reengineered cancer-fighting immune cells called CAR-T cells by moving an important widget from the cells' interior to the exterior. That's a weird move -- as weird as if they took a piece of the coffee-grinder's insides and glued it to the outside of the lid.
But their unusual strategy succeeded. As explained in a new study published recently in Nature, the team's inside-out innovation produced cancer-killing cells more likely to zero in on tumors and leave healthy tissues alone, a much-needed development in cancer immunotherapy.
"We didn't expect this to work at all," said Majzner, assistant professor of pediatrics. "We assumed you needed to use something that's on the cell surface to make a surface receptor."
CAR-T cells, a.k.a. chimeric antigen receptor T-cells, are immune cells engineered to fight cancer. To make them, doctors take immune cells from a patient's body and modify them in a lab, adding a synthetic receptor to their surfaces that recognizes a specific marker on the exterior of tumor cells. They then give the CAR-T cells back to the patient. The engineered cells travel through the blood, bind cancerous cells, and attack and destroy the cancer.
Targeting cancer, avoiding healthy tissue
CAR-T cells are used in a handful of FDA-approved cancer treatments that target specific forms of blood cancers originating from white blood cells known as B cells. While effective, these treatments have a catch: The CAR-T cells go after surface markers found abundantly on both healthy and cancerous B cells. The therapies get rid of cancer, but they also kill the patient's healthy B cells.
"You can live without B cells," Majzner said. "That's why it's worked."
But, because you can't safely give cells that attack, say, a patient's healthy liver or lung tissue, designing CAR-T cells to attack solid tumors in patients' organs has been tricky.
"Researchers have been struggling to find something that works well for solid tumors," said Tousley, a life science researcher, noting that solid tumors' markers are shared with healthy tissues. "They don't have one distinct target that's really easy to go after."
A possible solution is to make CAR-T cells that attack only when they bind a specific combination of two varieties of cell-surface markers, since the surfaces of tumor cells may carry different combinations of molecules on their surfaces than healthy cells do. Until now, scientists have been limited by the fact that they've scarcely varied the molecular widgets used to assemble CAR-T cells, restricting their options for creating cells that switch on in response to combinations of signals. To get around this problem, Majzner and Tousley began tinkering with the inner workings of CAR-T cells.
A "domino chain" of protein signals
Cells have a lot of moving parts, including many proteins that convey signals from the surface of the cell to its interior. These proteins work like a chain of dominoes falling over. A receptor on the cell's surface detects the start of the message, then passes it along by inducing small molecular changes inside the cell. These changes cascade through a long sequence of distinct proteins, each of which causes a molecular modification to the next member of the lineup.
In their hunt for new ways to activate CAR-T cells, the scientists decided to embed some of the mid-sequence proteins into the cell membrane, linking them to the chimeric antigen receptors that bind markers on other cells, including cancer. Would this switch on the CAR-T cells' killing ability? Weirdly, said Tousley, it did.
The scientists then dug into the mechanics of exactly how the signaling gets activated. They figured out (with a lot of trial and error) how to make two types of engineered receptors each latch onto a different molecule on a cancer cell and then bind with each other. Together (and only together!) the complex activates the signal cascade to start the cells' cancer-killing activity.
The feat required many returns to the discoveries of the 1990s detailing cells' signal cascades.
"Aidan spent the COVID shutdown reading old papers that defined the basic biology of these molecules," Majzner said, noting that the early studies were done decades ago just for the sake of curiosity, not with any eye toward future cures. "Ultimately, we broke open a new form of cellular engineering that allows us to use the internal machinery of the cells in unexpected ways."
From curiosity to cancer therapy
Many of the team's early CAR-T cells had "leaky" activation, meaning the cells were activated by just one of the two molecules on cancer cell surfaces, molecules also found on healthy cells. In other words, the CAR-T cells' safeguard to target cancer-specific combinations of surface molecules wasn't quite working, and they could accidentally attack healthy cells.
The team had to make more than 100 variants of the synthetic receptors to eventually solve this problem, Tousley said.
"Getting to the point where the cells were switched on and consistently killing only cancer cells with both markers took over a year of engineering and generating different variants of CAR-T cells," he said.
Early tests in mice show that the CAR-T cells can indeed target solid tumors without hurting healthy tissues, though more research is needed to identify the best pairs of cell markers for the therapy to target in human cancers.
"Anyone who has gone through cancer knows that a lot of current treatments are devastating to the entire body," said Tousley. "We're trying to figure out ways to attack just the cancer and leave the rest of the body unharmed."
He's glad he stuck with the unconventional project.
"I liked this idea of, 'Can we do this differently than how it's traditionally done?'" said Tousley. "This is an unexplored field of chimeric antigen receptor design. The novelty was really fun and kept me hooked even when there were a lot of failures."
Photo by Design Cells