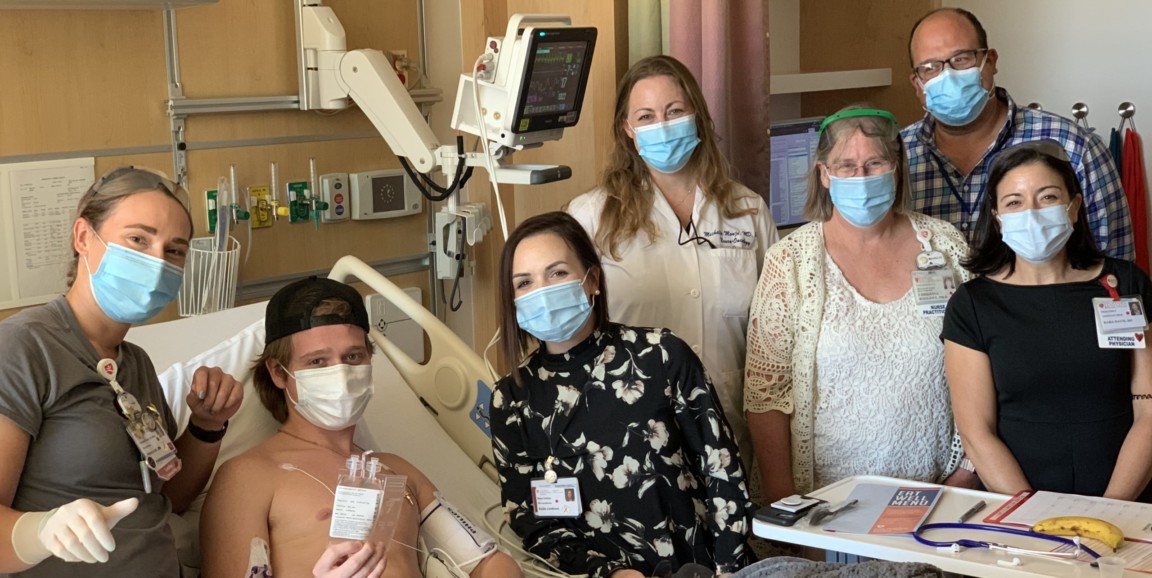
When Jace Ward came to Lucile Packard Children's Hospital Stanford in September 2020 to join a clinical trial for a novel therapy, he had been fighting a deadly brainstem tumor for more than a year. His diagnosis was diffuse intrinsic pontine glioma, or DIPG, which conventional cancer treatments can't cure. The disease has a five-year survival rate of less than one percent.
Today a group of Stanford scientists who have spent the past decade unlocking the glioma's secrets are publishing data in Nature from the trial Ward joined. He was one of the first four patients with diffuse intrinsic pontine glioma or a closely related cancer affecting the spinal cord to receive immune cells engineered to fight the disease.
Though all the trial patients died of their disease or its complications, three of them experienced significant clinical benefits from the engineered cells.
"These four patients are heroes," said study's principal investigator, pediatric neuro-oncologist Michelle Monje, MD, PhD. "They taught us so much, and that knowledge is already being applied to help other kids."
The FDA approved the use of engineered immune cells, also known as chimeric antigen receptor T-cells, or CAR-T cells, to treat blood cancers in 2017, but the technology has not previously succeeded against solid tumors.
Although the research team has yet to achieve a cure for this type of glioma, they consider their findings a milestone.
"We see significant anti-tumor activity with these CAR-T cell therapies in this dreaded disease," said cancer immunotherapy expert Crystal Mackall, MD, who shares senior authorship of the new research with Monje. The findings are a hopeful sign for many types of brain tumors, not just this one, she added. "It's a small data set, but the evidence is there."
The case of Jace Ward
Ward was the trial's second participant. His experience with the CAR-T cells was characterized by several important breakthroughs, including monthslong reversals of his neurological symptoms, Monje said. "The fact that there were responses, transient though they may have been, brings hope."
Ward was 20 when he was diagnosed with the glioma in early 2019, after a few weeks of disturbance in his peripheral vision. He and his family were shocked by the prognosis: Ward's neurologist in Kansas predicted he had six to nine months to live. He received an experimental chemotherapy agent on a compassionate-use basis and lived with the disease for almost 14 months before the opportunity to join the Stanford trial arose.
By the time he and his mom, Lisa, arrived at Stanford from their hometown of Wamego, Kansas, the tumor was progressing. Ward had started limping, could not use his right hand well, had weakness and loss of sensation on the left side of his face, and couldn't open his mouth wide enough to take bites of hamburgers or pizza, his favorite foods.

Ward knew the trial was unlikely to save his life. "He said, 'I know that I'm going to die, and I know this therapy will one day be the thing that cures other kids. Figure it out with me,'" Monje recalled. "This tough, football-playing 21-year-old said to me, 'I don't want it to be a 5-year-old who has to go first.'"
Ward saw his role as helping physicians understand the new treatment, his mom said. He and his family had been told that the trial carried significant risks.
"Jace said, 'Let me make the decision to do this,'" Lisa Ward said. "'It's not fair for parents to have to make this decision for their young child and live with regret. Let me go first.'"
Tumor donations for research
High-grade gliomas, a family of severe tumors, can occur at any time of life, and tend to develop in different regions of the brain depending on the age of the patient.
Among these are diffuse intrinsic pontine gliomas that occur in a few hundred new cases in the United States each year, mostly among school-aged children. Surgery isn't an option because the tumor cells grow intertwined with healthy brain cells in a critical region of the brainstem that's responsible for maintaining bodily functions such as breathing and heartbeat. A few chemotherapy drugs are under investigation, but none have been approved for the disease, and radiation buys only a short reprieve before the tumor worsens again.
When Monje launched her research career at Stanford Medicine in 2008, she and her colleagues decided to ask families whose children had the disease to donate their child's brain tumor after death. Eighty-seven families have done so, which enabled scientists to study the tumors' biology. Among the discoveries Monje's team made as a result is that the tumor cells form electrically active synapses with healthy brain cells and use those connections to drive their malignant growth.
Another big discovery came in 2018 when a student in Monje's lab characterized the molecular markers on the tumor cells' surfaces. The cells express vast quantities of a marker called GD2, the research showed. The molecule, a combination of lipids (fats) and sugars, is abundant on some other cancers. In fact, Mackall had already engineered CAR-T cells that could target GD2. Mackall's and Monje's teams collaborated on preclinical trials demonstrating that the engineered cells cleared diffuse intrinsic pontine glioma tumors in mice.
Engineering cells to fight cancer
Treating cancer with CAR-T cells involves removing immune cells called T-cells from the patient, engineering them in a lab to recognize cell markers abundant on tumors, then returning them to the patient. The altered cells include a protein -- the chimeric antigen receptor, or CAR -- that doesn't exist in nature.
The CAR protein binds the tumor and activates an immune response: The engineered T-cells multiply to create an armada of tumor fighters. These cells, and other immune cells they attract, attack and kill cancer cells, shrinking the tumor. The resulting immune response also includes surges of cytokines, inflammation-promoting molecules.
Although CAR-T cells can treat blood cancers, trials in solid tumors have faltered. One reason is geography: In blood cancers, malignant cells are distributed in blood and bone marrow, accessible to roving CAR-T cells.


But it's difficult for the cells to penetrate solid tumors. Additionally, the patient's immune system recognizes CAR proteins as foreign, and the body mounts an immune response to the therapy after the first dose. So one shot of the treatment is all that's effective, historically.
To try to boost the power of the engineered cells, Mackall's team developed approaches to prevent rapid exhaustion of CAR-T cells' replication ability. They've also fine-tuned the cells to respond only to high levels of molecular markers such as GD2, which keeps them from going after healthy cells with a few of the same markers.
"When you're creating a new therapy, it's more like invention than discovery," said Mackall, the Ernest and Amelia Gallo Family Professor of Pediatrics and Internal Medicine. "Inventions are iterative, with improvements over time. Now, 10 years into the CAR business, we know more about what these proteins need to be potent and safe."
The improved CAR-T cells showed promise in a mouse study that Mackall and Monje published in 2018. But it held warning signs: Inflammation in the tumor caused swelling in the brainstem of mice that received the CAR-T cells. Swelling in the brainstem can block drainage of fluid from around and within the brain, a dangerous situation.
So when the scientists moved into clinical trials, they built in precautions. For instance, before treatment, a surgeon implanted a device under the scalps of study participants to drain the cerebrospinal fluid that bathes the brain, allowing for pressure to be quickly lowered if swelling occurred. The researchers also planned to hospitalize all participants in intensive care after they received the engineered cells.
The advance planning paid off: Though patients did develop swelling in the tumor, usually around a week after getting CAR-T cells, the team treated it safely and effectively.
Children with brainstem tumors are extra-vulnerable to swelling because the tumor already takes up space inside their skulls, Monje said: "This space is like a cup that's nearly filled up, and then if you put in one drop, and another drop, it can overflow. Just a little bit tips over [into the danger zone] if it's a large tumor."
'I can't die, I'm busy'
When Ward joined the CAR-T cell trial, he was a junior at Kansas State University, studying entrepreneurship and pre-law. He was always willing to speak his mind and question authority figures, his mom said. After his diagnosis, Ward became a strong advocate for kids with diffuse intrinsic pontine glioma.
"Jace spoke at Congress, the National Institutes of Health and in rare-cancer virtual forums," Lisa Ward said. He was passionate about helping others access clinical trials, a daunting process that involves navigating complex eligibility requirements and often traveling cross-country.

Jace Ward worked on collaborative fundraising campaigns that raised more than $2.5 million to expand cancer research and treatment access. He also worked with his parents and others to launch a nonprofit that helps kids with brain tumors get expert opinions on clinical trials they can join.
"Jace would comfort his friends and family by saying, 'I can't die, I'm busy,'" Ward's mother said. "That became a mantra for him."
A week after Ward received his first infusion of CAR-T cells, given intravenously, he had fever and low blood pressure, signs of cytokine elevation, and his neurological symptoms worsened.
But by two weeks, he was experiencing a remarkable and unusual remission of neurological symptoms, opening his mouth fully and feeling more sensation on his face. His previously awkward, stiff-legged walking gait became almost normal. Within a month, his neurological exam was nearly normal, too.
He was thrilled to be able to open his mouth, Lisa Ward said. "He said that to eat a big hamburger or a slice of pizza was the best feeling in the world."
The scientists were excited for Ward as well as for what his results could mean for other patients. Not only is the tumor deadly, it severely debilitates patients during their lives, often causing paralysis, loss of hearing and ability to speak or swallow.
"To see a young man with rapidly progressive diffuse intrinsic pontine glioma regain an almost normal neurological exam is unheard-of," Monje said.
The researchers had suspected the tumors impaired, but didn't destroy, neural circuits, and that effective treatment might restore abilities the disease took away. Ward's response was the first proof.
"I felt for the first time that we were going to be able to cure this disease someday," Monje said.
Cancer therapy that will make kids feel better
"We didn't realize how dramatic the clinical improvement would be for these patients," Mackall said. Formal evaluations of patients' quality of life have been built into the next stages of the trial to thoroughly measure these benefits. "With cancer therapy, we're used to saying, 'It's toxic, you have to bear it,' but this is different. This therapy is going to make them feel better."
The researchers are also excited by growing evidence that CAR-T cell therapy may be especially useful for fighting many types of brain tumors. Mindful of the failures of other CAR-T cell trials for solid tumors, they decided to try something novel, infusing the cells directly into the cerebrospinal fluid. This approach had advantages: More CAR-T cells reached the tumor, and the cells were sequestered behind the blood-brain barrier, shielded from most of the patient's immune system.
Unlike studies with CAR-T cells in leukemia, where second doses are usually ineffective, the researchers found that patients could benefit from repeated doses of cells administered into the cerebrospinal fluid, likely because they escaped immune responses to the engineered cells. Patients also had less of the cytokines that cause undesirable side effects such as fever and low blood pressure.
When Ward's symptoms began to return a few months after his first infusion, the researchers infused CAR-T cells into his spinal fluid. Again, his tumor shrank, and he could walk and open his mouth widely.

"The walking was huge after his second infusion: He went in in a wheelchair and walked out of the hospital," said Lisa Ward, adding that her son went from not walking at all to walking four or more miles a day in less than a month. He was also able to fulfil a dream of going to the Super Bowl in February 2021 with his dad, Roger, to watch their beloved hometown team, the Kansas City Chiefs.
"It was so freeing for him, such a good glimmer of hope," Lisa Ward said.
Ward ultimately received five cell infusions over a 10-month period, which the researchers believe extended his life by several months. They plan to give up to 12 infusions to future trial participants, and are working to open clinical trials of CAR-T cells for other kinds of brain tumors, including glioblastoma in adults.
On June 30, 2021, while at home in Kansas, Ward experienced a severe hemorrhage in an area of his brain where the tumor had caused fragile blood vessels to grow. He was hospitalized in St. Louis and died July 3, leaving behind his grieving parents, brother, sister, sister-in-law and nephew.
"Do I wish he were here with me, never knowing what DIPG was? Of course," his mother said recently. But, she has gained a sense of peace and purpose from their work together as advocates for patients. "Jace's desire to help children during his fight, his real need to speak for them, gave me such a glimpse of the man he had become."
And she knows her son's role as a medical pioneer, the first brainstem tumor patient to be able to report back to his doctors on the benefits of CAR-T cells, meant a great deal to him. "He was able to tell them things all along the way that changed the trial," she said. "I think he found great purpose in that."
Photos courtesy of the Ward family. Top photo: On Sept. 23, 2020, Jace Ward received his first infusion of engineered immune cells at Lucile Packard Children's Hospital Stanford, surrounded by members of the clinical trial team, including, from left, nurse Rebecca Mitz; nurse Kayla Landrum; pediatric neurooncologist Michelle Monje, MD, PhD; clinical research nurse practitioner Christina Baggott, PhD; and pediatric oncologists Robbie Majzner, MD, and Kara Davis, DO.