A pair of closely related drugs, chloroquine and hydroxychloroquine, have gotten tons of attention, but so far, at best, mixed results in clinical trials for treating COVID-19.
These drugs -- primarily used to prevent and treat malaria and autoimmune diseases -- aren't necessarily going to be a panacea for this pandemic. But even if therapeutic expectations go unfulfilled, watching how they work in a lab dish can teach researchers a lot. Insights and tweaks from their observations could yield similar -- but more effective -- drugs, with their inadequacies ironed out and their SARS-CoV-2-fighting strengths honed to a sharp edge.
In Part 1 and Part 2 of a series called "What's a virus, anyway?" I described the general features of viruses and the specifics of how coronaviruses launch their invasion of our cells.
Here -- with hat tips to Stanford chemical engineer and subcellular-compartment spelunker Monther Abu-Remaileh, PhD, and virologist Jan Carette, PhD -- I describe one key way chloroquine grapples with SARS-CoV-2 within the nano-scale boxing ring that is a cell.
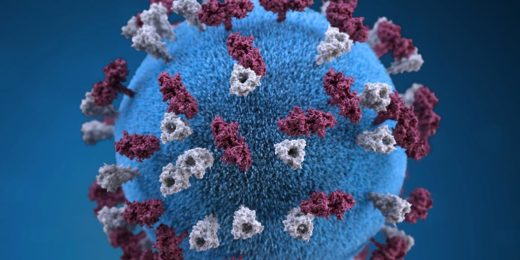
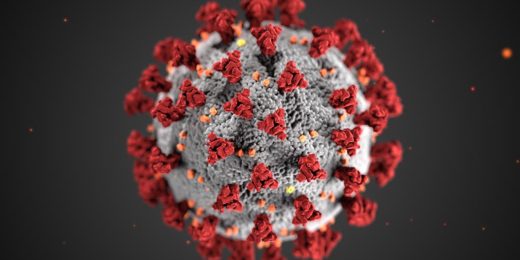
The attack begins with the coronavirus's initial assault on the cell's outer membrane. The coronavirus's envelope-anchored spike proteins, protruding like needles from a pincushion, latch onto receptors sitting on cells in the throat, lungs, heart, kidney, liver, brain, gut lining, stomach or blood vessels. This sets off a chain reaction that brings the virus's greasy envelope in contact with the cell's equally greasy outer membrane.
Grease loves grease. The viral envelope and cell membrane fuse, and a surrounding portion of the outer membrane caves in until it gets completely pinched off, forming an inbound, membrane-coated, liquid-centered capsule called an endosome inside the cell. (To visualize this, imagine yourself with a wad of bubble gum in your mouth, blowing an internal bubble by inhaling, and then swallowing it.)
Enclosed in this endosome is the viral particle that set the process in motion. The little devil has just hooked itself a free ride into the cell's inner sanctum.
At this point, the viral particle consists of its envelope, its capsid and its enclosed genome, a blueprint for all 29 proteins the virus needs. Among those proteins is one called a polymerase that, when the time is right, will function as a copying machine for the viral genome itself. SARS-CoV-2 needs its own special kind of polymerase because its genome is made of a substance called RNA instead of the more familiar DNA our genomes are made of. This means it can't be copied by the routine DNA polymerases our own cells use to copy our genomes.
But the endosome doesn't remain an endosome indefinitely, Abu-Remaileh told me. Its mission is to become a larger entity called a lysosome, or to fuse with an existing lysosome.
Lysosomes serve as cells' recycling factories, breaking down large biomolecules into their constituent building blocks for reuse. For this, they need an acidic environment, generated by protein pumps on endosomal and lysosomal surface membranes that force protons into these vesicles.
The building internal acidity activates endosomal enzymes that chew up the cloistered coronavirus's spike proteins. That brings the virus's envelope in contact with the endosomal membrane and enables their fusion.
The viral genome gets squirted out into the greater expanse of the cell, known as the cytoplasm. There, the viral genome finds and commandeers the raw materials and molecular machinery required to carry out its genetic instructions. That machinery furiously cranks out viral proteins -- including the customized polymerase SARS-CoV-2 needs to replicate its own genome. Copies of the genome and the virus's capsid proteins will soon be brought together and repackaged into viral progeny.
And here's where chloroquine comes in to defend the foundering cell. In a lab dish, anyway, chloroquine diffuses through both a cell's outer membrane and, next, that of the late endosome or lysosome, and steadily accumulates inside.
Chloroquine is a proton magnet: It grabs and holds onto protons, foiling the endosome's building acidity or diminishing the lysosome's. Without that requisite acidity, the viral-membrane spike proteins can't get chewed up, and the viral envelope can't make contact with the endosomal/lysosomal membrane. The virus remains locked in a prison of its own device, and the victim cell remains blithely unfazed.
That's what happens in a dish, anyway. As for what happens in living people, we'll have to wait and see. First, the cells in the dish aren't exactly the same as the cells in living tissues affected by SARS-CoV-2. Second, the environment surrounding, say, a lung cell in a living person's body is quite different from the one in a culture dish. And third, there's this thing called "side effects." You don't see those in a dish.
In the meantime, Abu-Remaileh, Carette and Stanford infectious-disease expert Catherine Blish, MD, PhD, are scrutinizing several ways chloroquine interferes with the viral life cycle, in a quest to find or build better drugs to treat COVID-19 and other diseases.
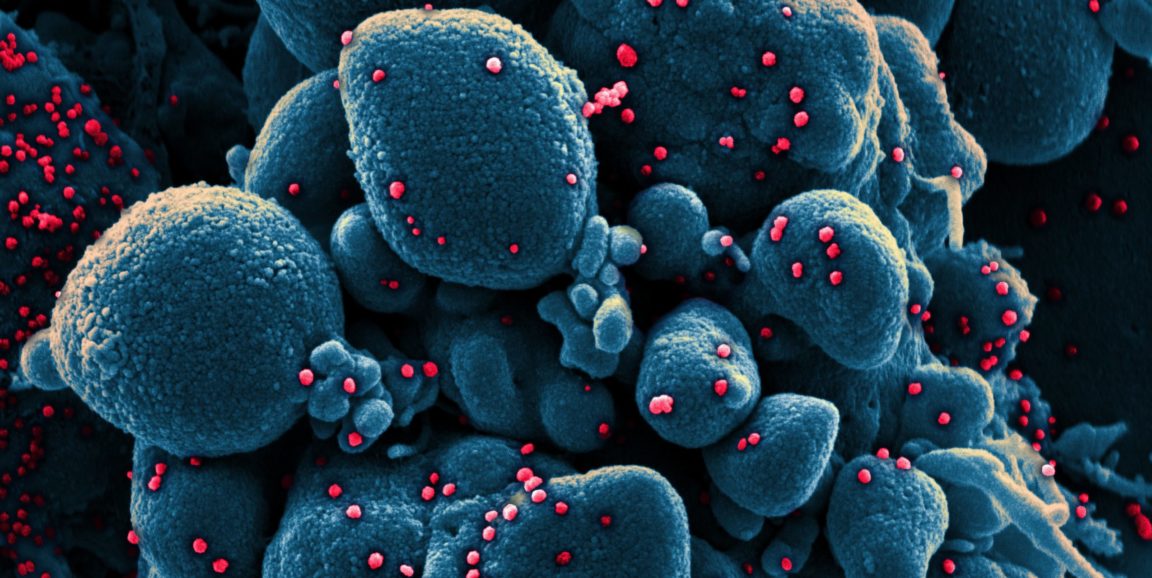
Photo by National Institute of Allergy and Infectious Diseases, NIH