The antiviral drug remdesivir has been approved for emergency use among hospitalized COVID-19 patients, and in recent studies, has shown promise as a treatment for the pandemic disease.
How exactly does remdesivir counter SARS-CoV-2 -- the coronavirus strain responsible for COVID-19?
With hat tips to virologist Jan Carette, PhD, geneticist Judith Frydman, PhD, and infectious-disease expert Bob Shafer, MD, let's follow the coronavirus downstream as it courses through its agenda within the infected cell.
Toward the end, we'll zero in on one of the virus's several Achilles heels, and we'll see how remdesivir could help -- and alas, why it may not be able to save the day on its own.
Invading a susceptible cell
Previously, I tracked the novel coronavirus's invasion of a susceptible cell. When we left off, SARS-CoV-2 had come riding into the cell like a Lilliputian aquanaut, stealthily stowed inside a little membrane-bound bubble called an endosome.
Within that endosome, the virus remains clad in its own membrane coat, or envelope, which (when things go right for the virus) fuses with the membrane of the surrounding endosome. The viral envelope's contents spill into the (relatively) vast surrounding cellular ocean, or cytosol, that occupies the space between the cell's nucleus and its outer membrane.
Chief among those contents is the virus's genome, which wriggles out of its self-imposed prison to pursue its destiny: It aspires to generate thousands of identical progeny that will eventually burst out through the cell's enclosing membrane and fan out to infect more cells.
Parenting a pack of viral progeny
That lonely single strand of RNA that is the virus's genome has a big job to do -- two, in fact, Frydman and Carette told me -- in order to bootstrap itself into parenting a pack of progeny. For one thing, it must replicate itself in entirety and in bulk, with each copy the potential seed of a new viral particle. For another, it must generate multiple partial copies of itself -- sawed-off snippets that serve as instruction guides telling the cell's protein-making machines, called ribosomes, how to manufacture the virus's more than two dozen proteins.
To do both of those things, the virus needs its own special kind of polymerase: a protein that acts as a copy machine for genetic material. Every living cell uses polymerases to copy its DNA-based genome, as well as to transcribe the resident genes along that genome into RNA-based instructions that ribosomes can read.
The SARS-CoV-2 genome, unlike ours, is made of RNA, so it's already ribosome-friendly; but replicating itself means making RNA copies of RNA. Our cells never need to do this, and they lack polymerases that can.
SARS-CoV-2's genome, though, does carry a gene coding for an RNA-to-RNA polymerase. If that lone RNA strand can find and latch onto a ribosome, the latter can translate the viral polymerase's genetic blueprint into a working polymerase. Fortunately for the virus, there can be as many as 10 million ribosomes in a single cell.
Errors in viral replication
Once made, the viral polymerase whirls into action, cranking out not only multiple copies of the full-length viral genome -- replication -- but also smaller sections, representing individual viral genes or groups of them. These smaller sections can clamber aboard ribosomes and command that they produce all of the proteins needed to assemble numerous new viral offspring.
This repertoire of newly created proteins includes, notably, more polymerase molecules. Each copy of the SARS-CoV-2 genome can be fed repeatedly through prolific polymerase molecules, generating myriad faithful reproductions of the initial RNA strand.
Well, mostly faithful. We all make mistakes, and the viral polymerase is no exception; actually it's pretty sloppy as polymerases go -- much more so than our own cells' polymerases, Carette and Frydman told me. So the copies of the initial viral genome -- and their copies -- are at risk of being riddled with copying errors, aka mutations.
However, coronavirus polymerases, including SARS-CoV-2's, come uniquely equipped with a sidekick "proofreader protein" that catches most of those errors. It chops out the wrongly inserted chemical component and gives the polymerase another, generally successful, stab at inserting the proper chemical unit into the growing RNA sequence.
How remdesivir disrupts the process
Here's where remdesivir could become important. It belongs to a class of antiviral drugs that work by posing as legitimate chemical building blocks of a DNA or RNA sequence. These poseurs get themselves stitched into the nascent strand and gum things up so badly that the polymerase stalls out or produces a defective product. Remdesivir has the virtue of not messing up our cells' own polymerases, said Shafer, who maintains a continuously updated database of results from trials of drugs targeting SARS-CoV-2.
"Now the virus is making a lot of rotten genomes that poison the viral replication process," Frydman told me. If its progeny are defective and unable to bust out and invade other cells throughout the body, the virus's mission is defeated -- and the patient gets better.
But while remdesivir is pretty good -- better than many other antivirals, anyway -- at faking out the viral polymerase's companion proofreader protein, it's far from perfect, Shafer said. Some intact viral-genome copies may still manage to get made, escape from the cell, and infect other cells -- mission accomplished.
Using remdesivir in combination with some still-sought, as-yet-undiscovered drug that could block the proofreader could turn out to be a more surefire strategy than remdesivir alone.
Barring that, it may well be that the most lethal aspect of SARS-CoV-2 is our own immune response to it.
Stay tuned.
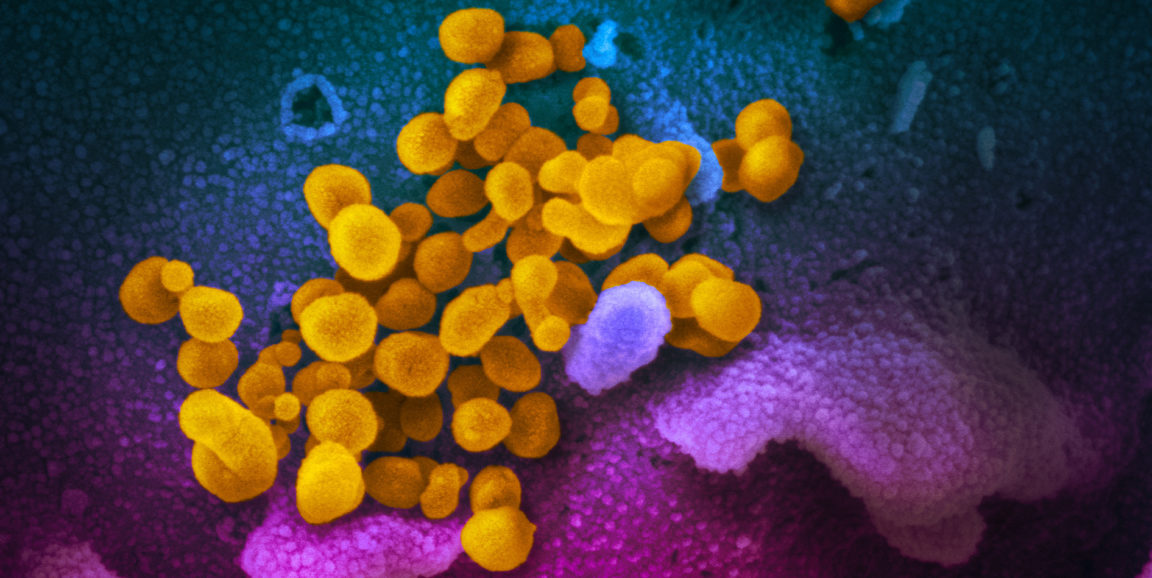
Image of SARS-CoV-2 emerging from the surface of cells cultured in the lab, courtesy of National Institute of Allergy and Infectious Diseases-Rocky Mountain Laboratories, NIH