What actually causes hearing loss? Are there new treatments that can restore hearing? Can it be reversed? How does air travel affect hearing loss?
Category: Surgery
PA student, a cancer survivor, rolls with the punches
She was a first-year PA student at Stanford Medicine when an MRI scan revealed that Melanie Shojinaga had a brain tumor.
A hunger to help people brought her to both surgery, cooking
Carlie Arbaugh is dedicated to both surgery and cooking because they demand meticulous attention to detail and the ability to think on your feet.
Do you sound like you? Gender-affirming voice therapy allows people to speak authentically
Why is it important to offer gender-affirming voice therapy or surgery? We spoke with experts on all sides of the equation.
Unconventional Paths: Rock climber turned trauma surgeon
Once a professional climber, Joe Forrester had a near-death experience that put him on a path to be a trauma surgeon at Stanford Medicine.
Contributing to greener Stanford Medicine operating rooms
Stanford Medicine sustainability experts work with researchers, doctors and environmental professionals to ensure greener operating rooms.
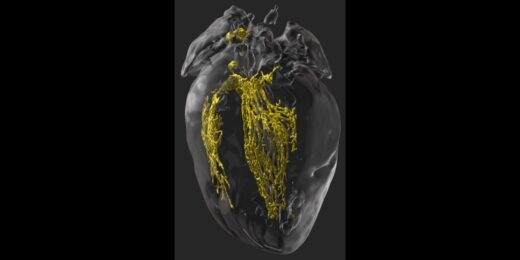
Making the invisible visible to improve heart surgery outcomes
Scientists find a way in mice to illuminate the cardiac conduction system during surgery to prevent unintended damage to healthy tissue.
On the field and in the clinic
Stanford Medicine orthopedic surgeon uses his skills to as head physician for the San Francisco 49ers football team
From Botox to headaches: The history and potential of migraine surgery
A Stanford plastic surgeon discusses a little-known treatment for migraines: surgery that involves decompressing a nerve.
A blood test to predict surgical complications?
Researchers create a blood test to predict a patient's risk for surgical site complications, such as infection.
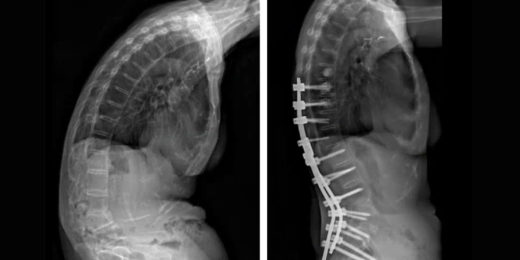
Orthopaedic surgery at Stanford helps woman stand upright
Lilly Lee's back was severely bent forward because of a spinal condition. Surgeon Serena Hu straightened it.
A serious gamed-based approach to assessing surgical residents
Researchers at Stanford Medicine have created a computer game to better educate medical students diagnosing patients during surgery.
Can major surgery increase risk for Alzheimer’s disease?
During cardiac surgery, patients’ blood levels of a substance highly predictive of Alzheimer’s disease jumped more than 5-fold.
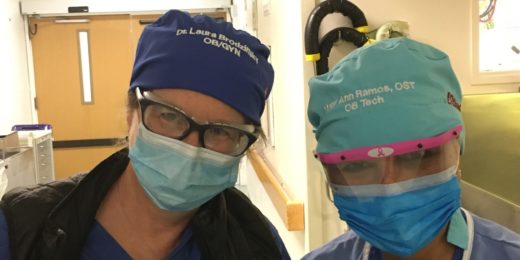
Names on surgical caps boost communication during C-sections, study finds
Wearing caps labeled with names and roles made it easier for everyone in the operating room to communicate during C-sections, a Stanford study found.
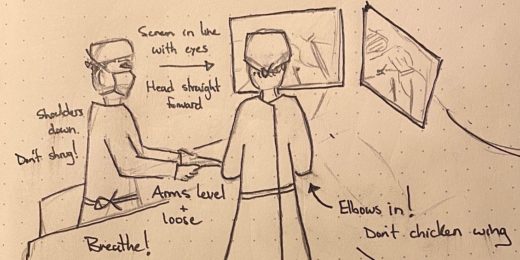
Surgical sketches help Stanford surgeon practice, teach
Graeme Rosenberg's illustrations, shared in classes he teaches and on social media, are resonating with fellow surgeons at Stanford and beyond.
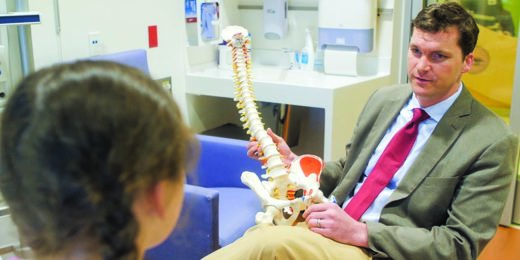
Stanford surgeon studies how to improve scoliosis treatment
Stanford researchers have several projects underway to improve imaging techniques, bracing treatment and surgeries for kids and teens with scoliosis.